The medical technology (MedTech) sector has traditionally been one of the strongest and most competitive sectors for innovation. Unsurprisingly, this has translated into large patent filing numbers worldwide, and a distinct upward trend since the global pandemic.1
Medical device technology forms one area of the MedTech sector. The IP generated from a new medical device is truly multidisciplinary, moving beyond IP in the hardware itself. IP from different disciplines is treated differently by patent systems worldwide. In view of this, it is important for innovators to be aware of exactly what IP they are generating so that they are able to make critical decisions regarding how best to protect their device.
This article provides a brief summary on the kind of IP that could be generated during medical device design and manufacture, and some general considerations regarding IP protection that innovators should be aware of depending on the nature of their medical device.
Types of medical devices
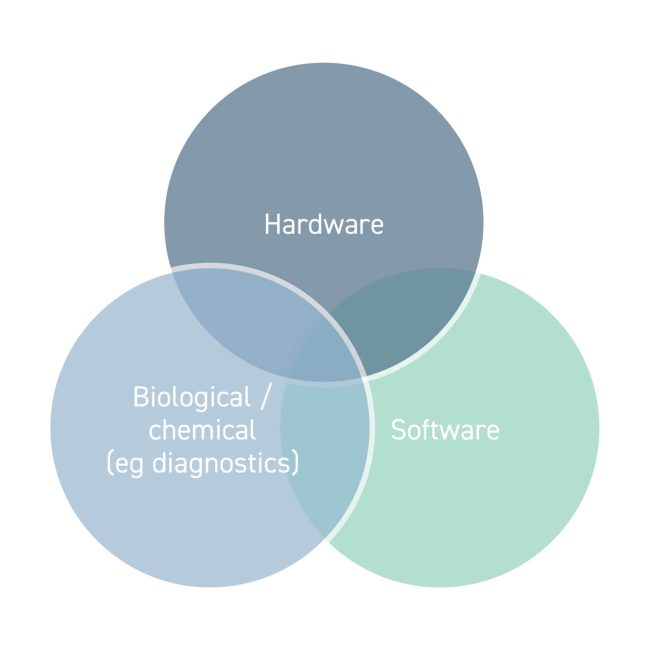
Hardware
The hardware of medical device technology is immediately apparent and historically the focus of this sector. Syringes, surgical equipment and dental tools are examples of this category.
Patents can be sought to protect the unique characteristics of the hardware and the mechanics of how the medical device works. These characteristics are well suited for patent protection being that they are tangible, vendible products that are often relatively easy to copy, and are going to end up in the hands of both the public and competitors.
Protecting the product itself is always the preferred and most powerful form of protection available. It provides an innovator a direct path to an infringing third party that is manufacturing, selling and/or importing the product into the protected jurisdiction.
Devices with chemical or biological features
Where a device has chemical or biological components, for example, for sensing or performing assays, the question arises whether the invention lies in the chemical or biological components, the chemical or biological components combined with the hardware of the device, and/or a new method, such as a method of diagnosis. Devices in this category include the SARS-CoV-2 rapid antigen tests that are now so well known for detecting COVID-19.
Devices containing chemical and biological features can also be the subject of protection, either by directing the patent claims to the chemical or biological features alone, or to those features in combination with the hardware. Transdermal patches and other devices with polymers to facilitate drug delivery are often protected in this way.
Diagnostic methods are patentable in some parts of the world and in some cases can provide more valuable IP than the patent for the diagnostic device itself.
For some sensing and diagnostic inventions, the hardware is not patentable and the focus must be on the chemistry or biology. This can occur when the hardware housing the chemical or biological feature is routine.
For diagnostic patents, additional effort is required to identify the expected infringers and ensure that the patent claims are crafted to capture these infringers. It is easy to draw a straight line to a party that infringes patented hardware. However, patented methods can present more of a challenge. There can be an infringer performing the diagnostic method, and another infringer performing a medical treatment in accordance with the diagnosis. In some circumstances, the party actually infringing a patented method may be a clinician or end-user – i.e. your prospective customers. These types of infringers are protected in some jurisdictions. Therefore, how your patent is crafted is critical in being able to bring action against the ‘right’ party.
Software inventions
A real growth area in MedTech is the utilisation of software, particularly the growing area of AI, to facilitate development or improvement in medical devices, as well as fast tracking medical diagnostics.
The patent system provides one way to protect such software innovations, be it in combination with the medical device hardware, or in its own right. However, depending on the exact nature of the invention, challenges may present themselves as one navigates the patenting laws of different jurisdictions – it can be difficult to obtain commercially valuable protection in some jurisdictions for software related inventions.
Given software innovations are often defined based on what the software is doing (e.g. a series of method steps), questions regarding who in fact would infringe your patent are relevant to think about with such inventions, as well as the even more pertinent question of how you would know if your patent is being infringed. Unlike a product, for example, that can be easily inspected and analysed, proving exactly what particular software is doing can be difficult and therefore increase the burden on the innovator to prove infringement.
As part of a diverse and robust IP strategy, it is important to be in dialogue with your patent attorney about whether trade secret protection is a suitable strategy for certain software innovations, particularly for innovations that are not easily reverse engineered. However, the usual cautions apply with trade secret protection – it is not the low cost alternative to patent protection that some often think it is. It can often be significantly less costly to protect a software invention by patent than it is to put the rigorous measures in place to maintain a trade secret.
Considerations for innovators in medical device technology
There are various considerations medical device innovators should keep in mind when developing their IP strategy. The best outcomes will invariably arise when the innovator is working in lockstep with their patent attorney, particularly one that is live to the dynamic nature of the development and commercialisation pathway of MedTech innovations.
Some of the considerations medical device innovators should bear in mind are:
- What IP have my innovations generated?
- In what direction are my innovations likely to go in the medium to long term?
- Do I need separate patents for each aspect of my new innovation?
- What other forms of IP protection do I have available to me?
- What is my optimal IP strategy and does it marry up with my overall commercialisation strategy?
- Do I require cross-discipline expertise – do my innovations involve some combination of new hardware, new software, and new diagnostic methodologies?
- Is my patent crafted to capture the ‘right’ parties?
- What should I do with new IP generated, e.g. through development and optimisation of core innovation, innovations arising through clinical trials?
Our MedTech team has practical experience in preparing strategic approaches to gaining commercially relevant protection for MedTech inventions across various disciplines, including medical devices. For further information, please contact the authors.